
The results suggest that the vaccine is about 91% effective in preventing COVID-19 in this age group.įor kids ages 12 through 15, the FDA reviewed a vaccine study of more than 2,200 U.S. Among 663 children given the placebo, there were 16 cases of COVID-19. Among 1,305 children given the vaccine, there were three cases of COVID-19. None of the children in this analysis had been diagnosed with COVID-19 before.

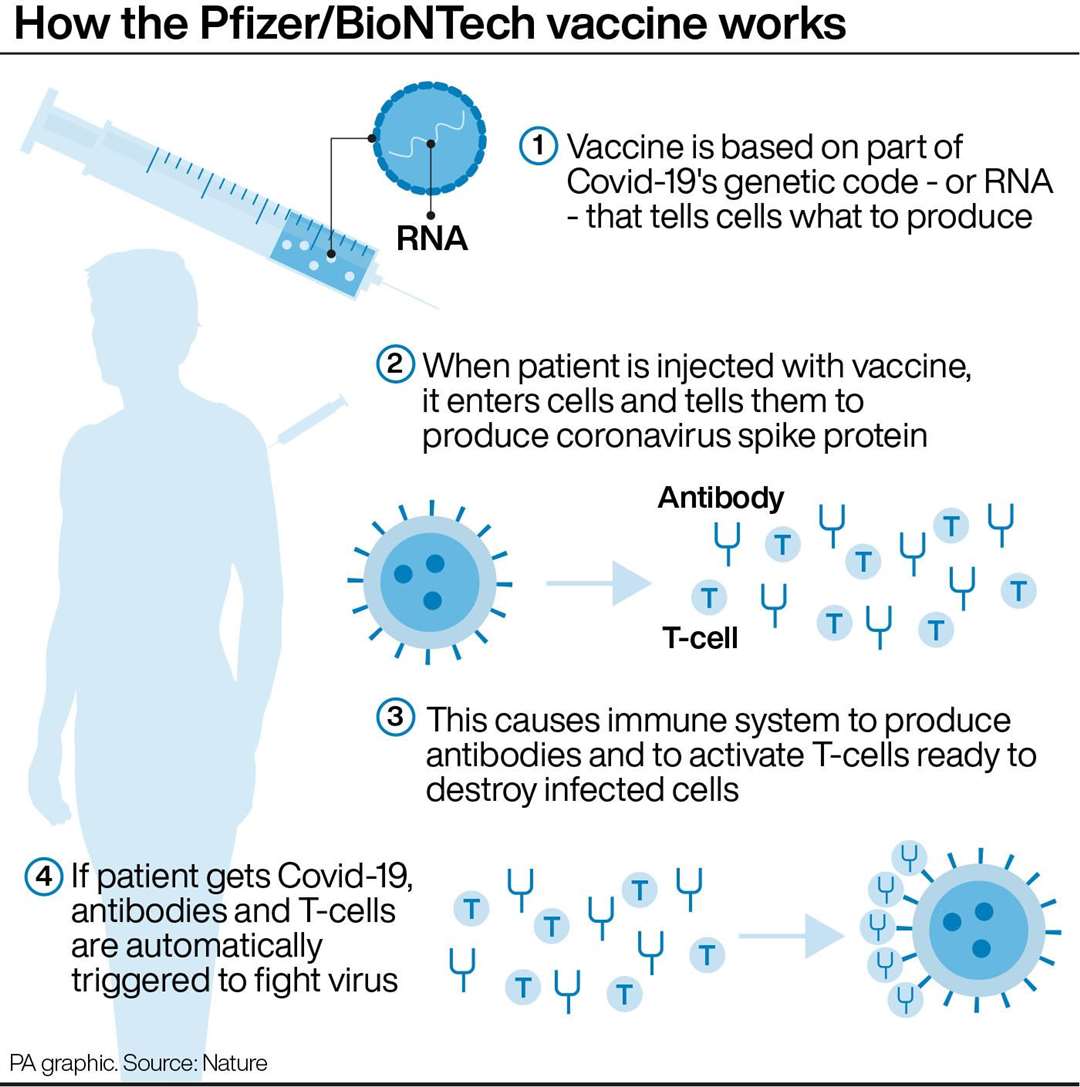
The FDA also took an early look at COVID-19 cases that happened one week after children were given a second dose of the vaccine. People age 12 and older who got the Novavax COVID-19 vaccine can choose between the updated Pfizer-BioNTech and the updated Moderna COVID-19 vaccine boosters. People age 6 and older who got either vaccine can choose between the updated Pfizer-BioNTech and the updated Moderna COVID-19 vaccine boosters. People age 5 and older who got the Moderna COVID-19 vaccine can choose between the updated Pfizer-BioNTech and the updated Moderna COVID-19 vaccine boosters. The booster is based on the original virus strain and two omicron strains.Īge 5 or 6 and older. Kids ages 6 months through 5 years old who got the Pfizer-BioNTech Covid-19 vaccine can only get the updated Pfizer-BioNTech booster. The booster is based on the original virus strain and two omicron strains. Kids ages 6 months through 4 years old who got the Moderna COVID-19 vaccine can only get an updated, called bivalent, Moderna COVID-19 vaccine booster. People who recently had a positive COVID-19 test may think about waiting three months after their symptoms started to get the booster.Īges 6 months through 4 or 5 years old. But in general, people can get the booster shot at least two months after their last shot. These recommendations differ by age, what vaccines you have been given and the state of your immune system. Research suggests that getting a booster dose can decrease the risk of infection and severe illness with COVID-19. Booster dosesīooster doses can help people who are vaccinated and whose immune response weakened over time. For people age 12 and older, the additional shot should be given at least four weeks after the second shot for the Pfizer-BioNTech COVID-19 vaccine and Moderna COVID-19 vaccine. This shot should be given at least four weeks after the second shot for kids ages 5 through 11 for the Pfizer-BioNTech COVID-19 vaccine and the Moderna COVID-19 vaccine. People age 5 and older who have moderately or severely weakened immune systems should get additional doses of the Pfizer-BioNTech COVID-19 vaccine or the Moderna COVID-19 vaccine. The Centers for Disease Control and Prevention (CDC) now recommends that children ages 6 months to 5 years old who have a weakened immune system get an additional dose of the Moderna COVID-19 vaccine at least four weeks after their second shot. It might increase protection against COVID-19 and lower the risk of rare heart problems, such as myocarditis and pericarditis.Īn additional primary shot of a COVID-19 vaccine can help people who are vaccinated and might not have had a strong enough immune response. Waiting eight weeks between the first and second doses might be best for some people, especially males ages 12 to 39.
#Pfizer second dose timing cdc series#
This vaccine is a series of two shots, given 3 to 8 weeks apart. The FDA also has given emergency use authorization to a Novavax COVID-19 vaccine for people age 12 and older. It has the same amount of mRNA as the Moderna COVID-19 vaccine for people age 18 and older. This vaccine is a series of two shots, given 4 to 8 weeks apart. The FDA also has given emergency use authorization to a Moderna COVID-19 vaccine for people ages 12 through 17. The vaccine has the same amount of mRNA as the Pfizer-BioNTech COVID-19 vaccine for people age 16 and older. This vaccine involves two shots, given 3 to 8 weeks apart. The FDA has approved the Pfizer-BioNTech COVID-19 vaccine, now called Comirnaty, for people ages 12 through 17. This vaccine involves two shots, given 4 to 8 weeks apart.īoth vaccines have lower amounts of mRNA than the COVID-19 vaccines for people age 12 and older.Īges 12 through 17. The FDA also has given emergency use authorization to a Moderna COVID-19 vaccine for children ages 6 through 11. It has a lower amount of mRNA than the Pfizer-BioNTech COVID-19 vaccine used for people age 12 and older. The FDA has given emergency use authorization to a Pfizer-BioNTech COVID-19 vaccine for children ages 5 through 11. This vaccine is a series of two shots, given 4 to 8 weeks apart.īoth vaccines have lower amounts of messenger RNA, also called mRNA, than the mRNA COVID-19 vaccines for older children and adults.Īges 5 or 6 through 11. The FDA also has given emergency use authorization to a Moderna COVID-19 vaccine for children ages 6 months through 5 years old.


 0 kommentar(er)
0 kommentar(er)
